Разновидность наследственного овалоцитоза, известная как Юго-восточноазиатский овалоцитоз, встречается среди населения южной части Тихого океана. Эту патологию рассматривают как эволюционно сложившую-ся форму защиты от малярии. Овалоцитоз развивается в результате делеции 27 пар нуклеотидов в гене протеина полосы 3. При этом синтезируется особый ва-риант протеина с выпадением аминокислот в позиции 400–408 в участке свя-зывания N-терминального и первого трансмембранного доменов. Такой вари-ант протеина полосы 3 (вариант Мемфис I) неактивен в отношении транспор-та анионов. Несмотря на высокую частоту указанной делеции среди жителей Океании, не было найдено ни одного индивида, гомозиготного по этой делеции. Есть основания полагать, что гомозиготность по любой из мутаций, инактиви-рующей протеин полосы 3, является летальной.
Booth и соавт. [15], Daniels и соавт. [39], Smythe и соавт. [154] установили, что у жителей Меланезии снижена экспрессия многих эритроцитарных антиге-нов: Di b, Wr b (системы Diegо); S, s, U, En a (системы MN); D, C, e (системы Rh); Kp b (системы Kell); Jk a, Jk b (системы Кидд); Xg a (системы XG); Sc1 (системы Scianna); LW (системы Landsteiner – Wiener); Ge2, Ge3, Ge4 (системы Gerbich); I T, I F (системы Ii).
Супрессия перечисленных антигенов может быть результатом поломок в трансмембранных доменах протеина полосы 3, сказывающихся на интеграции различных мембранных структур. Уменьшение количества вещества D, C, c, E, LW, S, s и U происходит в связи с замедлением транспорта этих веществ
с поверхности мембраны (Beckmann и соавт. [12]). Могут иметь место боко-вые разрывы белковых комплексов внутри мембраны и другие дефекты, обу-словленные неполноценным белком полосы 3 (Daniels и соавт. [39], Smythe и
соавт. [154]).
678
Список литературы
а Неванлинна Х.Р. Распределение различных генетических маркеров в Финляндии и проявление их в Эстонии и Венгрии // Этногенез финно-угорских народов по данным антропологии. – М., 1974. – С. 84–96.
а Фатьянов С.А., Мороков В.А. Естественные аллоантитела к редкому эритроцитарному антигену Wr a (Wright) системы Diego // Иммунология. – 2004. – V. 9. – Suppl. 1. – C. 61.
а Фатьянов С.А., Мороков В.А., Тоинов А.А. Аллоантитела к редкому антигену Wr a(Wright) системы Diego // Вестник службы крови России. – 2004. – № 2. – С .43–46.
а Adams J., Broviac M., Brooks W. et al. An antibody, in a serum of Wr(a + ) individual, reacting with an antigen of very high frequency // Transfusion. – 1971. – V. 11. – P. 290–291.
а Ainsworth B.M., Fraser I.D., Poole G.D. Severe haemolytic anaemia due to anti-Wr b [Abstract] // 20-th Cong. Int. Soc. Blood Transfus., 1988. – P. 88.
а Allen F.H., Corcoran P.A. Blood groups of the Penobscot Indians // Amer. J. Phys. Anthrop. – 1960. – V. 18. – P. 109–114.
а Alves De Lima L.M., Berthier M.E. Characterization of an anti-Di b antibody causing
hemolytic disease in a newborn infant // Transfusion. – 1982. – V. 22. – P. 246–247.
8. Anstee D.J., Edwards P.A.W. Monoclonal antibodies to human erythrocytes // Eur.
J. Immunol. – 1982. – V. 12. – P. 228–232.
и Arriaga F., Palan F., Lopez T. et al. Anti-Wr a in newborn twins // Transfusion. – 1991. –
31. – P. 381–382.
и Auffray I., Marfatia S., de Jong K. et al. Glycophorin A dimerization and band 3 interaction during erythroid membrane biogenesis: in vivo studies in human glycophorin A transgenic mice // Blood. – 2001. – V. 97. – P. 2872–2878.
и Beckman R., Smythe J.S., Anstee D.J., Tanner M.J.A. Functional cell surface expression of band 3, the human red blood cell anion exchange protein (AE1), in K562 erythroleukemia cells: band 3 enhances the cell surface reactivity of Rh antigens // Blood. – 1998. – V. 92. –
4428–4438.
и Beckmann R., Smythe J.S., Anstee D.J., Tanner M.J.A. Coexpression of band 3 mutants and Rh polypeptides: differential effects of band 3 on the expression of the Rh complex containing D polypeptide and the Rh complex containing CcEe polypeptide // Blood. – 2001. – V. 97. – P.2 496–2505.
и Better P.J., Ford D.S., Frascarelli A., Stern D.A. Confirmation of anti-ELO as a cause of haemolytic disease of the newborn // Vox Sang. – 1993. – V. 65. – P. 70.
и Biro V., Garratty G., Johnson C.L., Marsh W.L. Depressed blood group antigens on red cells from a Mexican donor // Transfusion. – 1996. – V. 23. – P. 65–66.
и Booth P.B., Serjeantson S., Woodfield D.G., Amato D. Selective depression of blood group antigens associated with hereditary ovalocytosis among Melanesians // Vox Sang. – 1977. –
О 32. – P. 99–110.
и Bruce L.J., Anstee D.J., Spring F.A., Tanner M.J.A. Band 3 Memphis variant II: altered stilbene disulphonate binding and the Diego (Di a) blood group antigen are associated with the human erythrocyte band 3 mutation Pro854→Leu // J. Biol. Chem. – 1994. – V. 269. –
16155–16158.
с Bruce L.J., Ring S.M., Anstee D.J. et al. Changes in the blood group Wright antigens are associated with a mutation at amino acid 658 in human erythrocyte band 3 and glycophorin A under certain conditions // Blood. – 1995. – V. 85. – P. 541–547.
с Bruce L.J., Tanner M.J.A. Erythroid band 3 variants and disease // Bailliere’s Best Pract. Res. Clin Haematol. – 1999. – V. 12. – P. 637–654.
с Bruce L.J., Unwin R.J., Wrong O., Tanner M.J.A. The association between familial distal renal tubular acidosis and mutations in the red cell anion exchanger (band 3. AE1) gene // Biochem. Cell. Biol. – 1998. – V. 76. – P. 723–728.
679
и Bruce L.J., Wrong O., Toye A.M. et al. Band 3 mutations, renal tubular acidosis and South East Asian ovalocytosis in Malaysia and Papua New Guinea: loss of up to 95 % band 3 transport in red cells // Biochem J. – 2000. – V. 350. – P. 41–51.
и Bruce L.J., Zelinski T., Ridgwell K., Tanner M.J.A. The low-incidence blood group antigen, Wd a, is associated with the substitution Val557→Met in human erythrocyte band 3 (AE1) // Vox Sang. – 1996. – V. 71. – P. 118–120.
и Byrne K.M., Byrne P.C. Review: other blood group systems – Diego, Yt, Xg, Scianna, Dombrock, Colton, Landsteiner – Wiener, and Indian // Immunohematology. – 2004. –
20. – P. 50–59.
и Cann H.M., Van West B., Barnett C.R. Genetics of Diego blood groups in Guatemala Indians: use of antiserums to Diego a and Diego b antigens // Science. – 1968. – V. 162. –
1391–1392.
В Castilho L., Rios M., Soares M. et al. High frequency of the DI*A allele associated with the mutationLys56Glu in Amazonian Indians [Abstract] // Blood. – 1999. – V. 94 (Suppl.1). –
458a.
В Chaves M.A., Leak M.R., Poole J., Giles C.M. A new low-frequency antigen BOW (Bowyer) // Vox Sang. – 1988. – V. 55. – P. 241–243.
В Che A., Cherry R.J. Loss of rotation mobility of band 3 proteins in human erythrocyte membranes induced by antibodies to glycophorin A // Biophys. J. – 1995. –V. 68. –
1881–1887.
В Cleghorn T.E. A ‘new’ human blood group antigen, Sw a // Nature. – 1959. – V. 184. –
1324.
В Cleghorn T.E. The frequency of the Wr a, By and M g blood group antigens in blood donors in the South of England // Vox Sang. –1960. – V. 5. – P. 556–560.
В Coghlan G., Crow M., Spruell P. et al. A ‘new’ low-incidence red cell antigen WARR: Unique to native Americans? // Vox Sang. – 1995. – V. 68. – P. 187–190.
В Coghlan G., Green C., Lubenko A. et al. Low-incidence red cell antigen ELO (700.51): evidence for exclusion from thirteen blood group systems // Vox Sang. – 1993. – V. 64. –
240–243.
В Contreras M., Lubenko A., Armitage S. et al. Frequency and inheritance of the Bx a (Box) antigen // Vox Sang. – 1980. – V. 39. – P. 225–228.
В Contreras M., Stebbing B., Mallory D.M. et al. The Redelberger antigen Rb a // Vox Sang. – 1978. – V. 35. – P. 397–400.
В Contreras M., Teesdale P., Moulds M. et al. Sw a: a subdivision // Vox Sang. – 1987. –
52. – P. 115–119.
и Dahr W., Schurt K.H., Arndt-Hansen A. et al. A novel phenotype within the Wright blood group collection [Abstract] // Transfusion. – 1992. – V. 32 (Suppl.). – 55S.
и Dahr W., Wilkinson S., Issitt P.D. et al. High frequency antigens of human erythrocyte membrane sialoglycoproteins. III. Studies on the En aFR, Wr b and Wr a antigens // Biol. Chem. Hoppe-Seyler – 1986. – V. 367. – P. 1033–1045.
и Daniels G.L. Effect of enzymes on and chemical modifications of high-frequency red cell antigens // Immunohematology. – 1992. – V. 8. – P. 53–57.
и Daniels G.L. Human Blood Groups. – 2-nd ed. – Oxford: Blackwell Science, 2002. – 560 p.
и Daniels G.L., Green C. Expression of red cell surface antigens during erythropoesis // Vox Sang. – 2000. – V. 78 (Suppl. 1). – P. 149–153.
и Daniels G.L., Johnson P.H., Coetzer T.L. et al. Depressed Gerbich (glycophorin C / D) red cell antigens associated with Southeast Asian ovalocytosis (SAO) in South African kindred [Abstract] // 24-th Cong. Int. Soc. Blood Transfus. – Makuhari, Japan., 1996. – P.110.
и Dankbar D.T., Pierse S.R., Issitt P.D. et al. Fatal intravascular hemolysis associated with auto anti-Wr b [Abstract] // Transfusion. – 1987. – V. 27. – P. 534.
680
13. Daw E. Haemolytic disease of the newborn due to the Wright antigen // J. Obstet. Gynaec. – 1971. – V. 78. – P. 377–378.
14. Edwards-Moulds J.M., Alperin J.B. Studies of the Diego blood groups among Mexican-Americans // Transfusion. – 1986. – V. 26. – P. 234–236.
15. Eriksson A.W., Frants R.R. Studies on blood groups in the Komi (Zyrians) // Suomen Antropol. Souran. Toimituksia 4, Helsinki, 1978. – P. 14–24.
16. Feller C.W., Shenker L., Scott E.P., Marsh W.L. An anti-Diego b (Di b) antibody occurring during pregnancy // Transfusion. – 1970. – V. 10. – P. 279–280.
17. Ford D.S., Stern D.A., Hawksworth D.N. et al. Haemolytic disease of the newborn probably due to anti-ELO, an antibody to low frequency red cell antigen // Vox Sang. – 1992. – V. 62. – P. 169–172.
18. Fujinaga J., Tang X.-B., Casey J.R. Topology of the membrane domain of human erythrocyte anion exchange protein, AE1 // J. Biol. Chem. – 1999. – V. 274, – P. 6626–6633.
19. Furuhjelm U., Nevanlinna H.R., Pirkola A. A second Finnish En(a −) propositus with anti-En a // Vox Sang. – 1973. – V. 24. – P. 545–549.
20. Gahmberg C.G., Andersson L.C. K562: a human leukemia cell line with erythroid features // Semin. Hemat. – 1981. – V. 18. – P. 72–77.
21. Gardner B., Parsons S.F., Merry A.H., Anstee D.J. Epitopes on sialoglycoprotein α: evidence for heterogeneity in the molecule // Immunology. – 1989. – V. 68. – P. 283–289.
22. Goldfinger D., Zwicker H., Belkin G.A., Issitt P.D. An autoantibody with anti-Wr b specificity in a patient with warm autoimmune hemolytic anemia // Transfusion. – 1975. – V. 15. – P. 351–352.
23. Graninger W. Anti-Di a and the Di a blood group: antigen found in Austrian family // Vox Sang. – 1976. – V. 31. – P. 13–135.
24. Greendyke R.M., Banzhaf J.C. Occurence of anti-Wr a in blood donors and in selected patient groups, with a note on the incidence of Wr a antigen // Transfusion. – 1977. – V. 17. – P. 621–624.
25. Groves J.D., Tanner M.J.A. Glycophorin A facilitates the expression of human Band 3-mediated anion transport in Xenopus oocytes // J. Biol. Chem. – 1992. – V. 267. – P. 22163–22170.
26. Habash J., Devenish A., Macdonald S. et al. A further example of anti-Di b not causing hemolytic disease of the newborn // Vox Sang. –1991. – V. 61. – P. 77.
27. Hardman J.T., Beck M.L. Hemagglutination in capillaries: Correlation with blood group specificity and IgG subclass // Transfusion. – 1981. – V. 21. – P. 87–88.
28. Harris P.A., De la Vega M.S., Clinton B.A., Miller W.V. Positive direct antiglobulin test due to anti-Fr a in a newborn infant // Transfusion. – 1983. – V. 23. – P. 394–395.
29. Hassoun H., Hanada T., Lutchman M. et al. Complete deficiency of glycophorin A in red blood cells from the mice with targeted inactivation of the band 3 (AE1) gene // Blood. – 1998. – V. 91. – P. 2146–2151.
30. Hinckley M.F., Huestis D.W. An immediate hemolytic transfusion reaction apparently caused by anti-Di a // Rev. Franc. Transfus. Immunohemat. – 1979. – V. 22. – P. 581–585.
31. Holman C.A. A new rare human blood group antigen (Wr a) // Lancet. – 1953. – V. ii. – P. 119.
32. Hsu L., Morrison M. A new variant of the anion transport protein in human erythrocytes // Biochemistry. – 1985. – V. 24. – P. 3086–3090.
33. Huang C.-H., Reid M.E., Xie S.-S., Blumenfeld O.O. Human red blood cell Wright antigens: a genetic and evolutionary perspective on glycophorine A-band 3 interaction // Blood. – 1996. – V. 87. – P. 3942–3947.
34. Ideguchi H., Okubo K., Ishikawa A. et al. Band 3-Memphis is associated with a lower transport rate of phosphoennolpyruvate // Brit. J. Haemat. – 1992. – V. 82. – P. 122–125.
681
32. Inaba M., Yawata A., Koshino I. et al. Defective anion transport and market spherocytosis and membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation // J. Clin. Invest. – 1996. – V. 97. – P. 1804–1817.
33. Ishimori T., Fukumoto Y., Abe K. et al. Rare Diego blood group phenotype Di(a +b −). I. Anti-Di b causing hemolytic disease of the newborn // Vox Sang. –1976. – V. 31. – P. 61–63.
34. Issitt P.D., Anstee D.J. Applied Blood Group Serology. – 4-th ed. – Durham, NC, USA: Montgomery Sc. Publ., 1998. – 1208 p.
35. Issitt P.D., Combs M.R., Allen J., Melroy-Carawan H. Anti-Di b as a red cell autoantibody // Transfusion. – 1996. – V. 36. – P. 802–804.
36. Issitt P.D., Pavone B.G., Goldfinger D. et al. Anti-Wr b, and other autoantibodies responsible for positive direct antiglobulin tests in 150 individuals // Brit. J. Haemat. – 1976. – V. 34. –
№ 5–18.
37. Issitt P.D., Pavone B.G., Goldfinger D., Zwicker H. An En(a −) red cell sample that types as Wr(a −b −) // Transfusion. – 1975. – V. 15. – P. 353–355.
38. Issitt P.D., Pavone B.G., Wagstaff W., Goldfinger D. The phenotypes En(a −), Wr(a −b −), and En(a + ), Wr(a +b −), and further studies on the Wright and En blood group systems // Transfusion. – 1976. – V. 16. – P. 396–407.
39. Issitt P.D., Wren M.R., Rueda E., Maltz M. Red cell antigens in Hispanic blood donors // Transfusion. – 1987. – V. 27. – P. 117.
40. Jarolim P., Murray J.L., Rubin H.L. et al. A Thr552→Ile substitution in erythroid band 3 gives rise to the Warrior blood group antigen // Transfusion. – 1997. – V. 37. – P. 398–405.
41. Jarolim P., Murray J.L., Rubin H.L. et al. Blood group antigens Rb a, Tr a, and Wd a are located in the third ectoplasmic loop of erythroid band 3 // Transfusion. – 1997. – V. 37. –
№ 607–615.
42. Jarolim P., Reid M.E. Substitution 480Glu→Lys in erythroid band 3 underlies the Fr a blood group antigen and supports the existence of the second ectoplasmic loop of band 3 [Abstract] // Blood. – 2000. – V. 96. – P. 593a.
43. Jarolim P., Rubin H.L., Moulds J.M. Multiple molecular mechanisms resulting in the Di(a +b −) phenotype [Abstract] // Transfusion. – 1996. – V. 36 (Suppl.1). – 49S.
44. Jarolim P., Rubin H.L., Zakova D. et al. Characterization of seven low incidence blood group antigens carried by erythrocyte band 3 protein // Blood. – 1998. – V. 92. – P. 4836–4843.
45. Jarolim P., Rubin H.L., Zhai S. et al. Band 3 Memphis: a widespread polymorphism with abnormal electrophoretic mobility of erythrocyte band 3 protein caused by substitution AAG→GAG (Lys→Glu) in codon 56 // Blood. – 1992. – V. 80. – P. 1592–1598.
46. Jorgensen J., Jakobsen L. Erythroblastosis fetalis caused by anti-Wr a(Wright) // Vox Sang. – 1974. – V. 27. – P. 478–479.
47. Kaita H., Lewis M., McAlpine P.J. Exclusion of the red blood cell antigen Fr a from the Colton blood group system // Transfusion. – 1980. – V. 20. – P. 217.
48. Kaita H., Lubenko A., Moulds M., Lewis M. A serologic relationship[ among the NFLD, BOW and Wu red cell antigens // Transfusion. – 1992. – V. 32. – P. 845–847.
49. Kay M.M.B. Cellular and molecular biology of senescent cell antigen // G. Garratty, ed. Immunobiology of Transfusion Medicine. – N.Y.: Marcel Dekker, 1997. – P. 173–198.
50. Kollert-Jons A., Wagner S., Hubner S. et al. Anion exanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus // Amer. J. Physiol. – 1993. –
265. – P. 813–821.
108. Kornstad L. A rare blood group antigen Ol a (Oldeide), associated with weak Rh antigens // Vox Sang. – 1986. – V. 50. – P. 235–239.
109. Kornstad L. Some observations on the Wright blood group system // Vox Sang. –1961. –
// 6. – P. 129–135.
682
90. Kornstad L., Brocteur J. A new, rare blood group antigen, Mo a (Moen) [Abstract] // Joint. Cong. Int. Soc. Blood Transfus. Am. Assoc. Blood Banks, 1972. – P. 58.
91. Kornstad L., Howell P., Jorgensen J. et al. The rare blood group antigen, Wu // Vox Sang. – 1976. – V. 31. – P. 337–343.
92. Kornstad L., Jerne D., Tippett P. The Haakestad antigen is identical with the Hov antigen // Vox Sang. – 1987. – V. 52. – P. 120–122.
93. Kornstad L., Kout M., Larsen A.M.H., Orjasaeter H. A rare blood group antigen, Jn a // Vox Sang. – 1967. – V. 13. – P. 165–170.
94. Kout M. The incidence of the C W, M g and Wr a agglutinogens in the population of Prague // Vox Sang. –1962. – V. 7. – P. 242–244.
95. Kusnierz-Alejska G., Bochenek S. Haemolytic disease of the newborn due to anti-Di a and incidence of the Di a antigen in Poland // Vox Sang. – 1992. – V. 62. – P. 124–126.
96. Lang N.A., Moulds M.K., Coghlan G.E. The Rebelberger antigen: a family study, a family story // Immunohematology. – 2006. – V. 22. – P. 48–51.
97. Langley J.W., Issitt P.D., Anstee D.J. et al. Another individual (J.R.) whose red blood cells appears to carry a hybrid MNSs sialoglycoprotein // Transfusion. – 1981. – V. 21. –
15–24.
98. Layrisse M., Arends T. The ‘Diego’ blood factor distribution: genetic, clinical and anthropological significance // Proc. 6-th Congr. Int. Soc. Blood Transfus., 1958. –
114–116.
99. Layrisse M., Arends T. The Diego blood factor in Chinese and Japanese // Nature. – 1956. –
177. – P. 1083–1084.
154. Layrisse M., Arends T. The Diego blood factor in Negroid populations // Nature. – 1957. –
// 179. – P. 478–479.
155. Layrisse M., Arends T., Dominguez Sisico R. Nuevo grupo sanguineo encontrado en descendientes de Indios // Acta Med. Venezolana. – 1955. – V. 3. – P. 132–138.
156. Layrisse M., Sanger R., Race R.R. The inheritance of the antigen Di a: evidence for its independence of other blood group systems // Amer. J. Hum. Genet. – 1959. – V. 11. –
17–25.
181. Leddy J.P., Wilkinson S.L., Kissel G.E. et al. Erythrocyte membrane proteins reactive with IgG (warm-reacting) anti-red blood autoantibodies. II. Antibodies coprecipitating band 3 and glycophorin A // Blood. – 1994. – V. 84. – P. 650–656.
182. Levine P., Robinson E.A. Some observations of the new human blood factor Di a // Blood. – 1957. – V. 12. – P. 448–453.
183. Levine P., Robinson E.A., Layrisse M. et al. The Diego blood factor // Nature. –1956. –
J. 177. – P. 40–41.
184. Lewis M., Ayukawa H., Chown B., Levine P. The blood group antigen Diego in North American Indians and in Japanese // Nature. – 1956. – V. 177. – P. 1084.
185. Lewis M., Kaita H. A ‘new’ low incidence ‘Hutterite’ blood group antigen Waldner (Wd a) // Amer. J. Hum. Genet. – 1981. – V. 33. – P. 418–420.
186. Lewis M., Kaita H., Allderdice P.W. et al. A ‘new’ low incidence red cell antigen, NFLD // Hum. Genet. – 1984. – V. 67. – P. 270–271.
187. Lewis M., Kaita H., Chown B. et al. A family with the rare red cell antigens Wr a and ‘super Sd a’ // Vox Sang. –1973. – V. 25. – P. 336–340.
188. Lewis M., Kaita H., Chown B. The blood group of a Japanese population // Amer. J. Hum. Genet. – 1957. – V. 9. – P. 274–283.
189. Lewis M., Kaita H., McAlpine P.J. et al. A ‘new’ blood group antigen Fr a: incidence, inheritance and genetic linkage analysis // Vox Sang. – 1978. – V. 35. – P. 251–254.
190. Lewis M., Kaita H., Philipps S. et al. The Swann phenotype 700:4, -41; genetic studies // Vox Sang. – 1988. – V. 54. – P. 184–187.
683
28 Lewis M., Kaita H., Philipps S., McAlpine P.J. The low-incidence red cell antigen Wr a: genetic studies // Transfusion. – 1991. – V. 31. – P. 47–51.
29 Lin C.K., Mak K.H., Chan N.K. et al. Report on anti-Di b encountered in two Hong Kong Chinese // Immunohematology. – 1997. – V. 13. – P. 17–19.
30 Lin-Chu M., Broadberry R.E., Chang F.J. The distribution of blood group antigens and alloantibodies among Chinese in Taiwan // Transfusion. – 1988. – V. 28. – P. 350–352.
31 Liotta I., Purpura M., Dawes B.J., Giles C.M. Some data on the low frequency antigens Wr a and Bp a // Vox Sang. –1970. – V. 19. – P. 540–543.
32 Lubenko A., Contreras M. The incidence of hemolytic disease of the newborn attributable to anti-Wr a // Transfusion. – 1992. – V. 32. – P. 87–88.
33 Lux S.E., John K.M., Kopito R.R., Lodish H.F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1) // Proc. Nat. Acad. Sci. USA. – 1989. –
86. – P. 9089–9093.
34 McGuire D., Funkhouser J.W. A study on the Wright blood group system as found in a normal donor population [Abstract] // Transfusion. – 1967. – V. 7. – P. 385.
35 McManus K., Lupe K., Coghlan G., Zelinski T. An amino acid substitution in the putative second extracellular loop of RBC band 3 accounts for the Froese blood group polymorphism
V.Transfusion. – 2000. – V. 40. – P. 1246–1249.
36 McManus K., Pongoski J., Coghlan G., Zelinski T. Amino acid substitutions in human erythroid protein, band 3 account for the low-incidence antigens NFLD and BOW // Transfusion. – 2000. – V. 40. – P. 325–329.
37 Metaxas M.N., Metaxas-Buhler M. A Swiss family showing independent segregation of the Lutheran and Swann genes // Vox Sang. – 1976. – V. 31 (Suppl.1). – P. 39–43.
38 Metaxas M.N., Metaxas-Buhler M. Studies on the Wright blood group system // Vox Sang. –1963. – V. 8. – P. 707–716.
39 Miyazaki T., Sato S., Kato T., Ikeda H. Human anti-Di a monoclonal antibodies for mass screening // Immunohematology. – 2000. – V. 16. – P. 78–81.
40 Mollison P.L., Engelfriet P., Contreras M. Blood Transfusion in Clinical Medicine. –10-th ed. – Oxford: BSP, 1997. – 1033 p.
41 Moores P., Smart E., Marks M., Botha M.C. Wd(a + ) red blood cells in two sisters of a Heiom Khoisan family in Namibia // Hum. Hered. – 1990. – V. 40. – P. 257–261.
42 Moulds M., Kaita H., Kornstad L., Lubenko A. Evidence that the low-incidence antigen termed Wu (700.13) and Hov (700.38) are identical // Vox Sang. – 1992. – V. 62. – P. 53–54.
43 Mourant A.E., Kopec A.C., Domaniewska-Sobczak K. The Distribution of Human Blood Groups and Other Polymorphisms. – 2-nd. ed. – London: Oxford University Press, 1976.
44 Mueller T.J., Morrison M. Detection of a variant of protein 3, the major transmembrane protein of the human erythrocyte // J. Biol. Chem. – 1977. – V. 252. – P. 6573–6576.
45 Nakajima H., Hayakawa Z., Ito H. A new example of anti-Di b found in a Japanese woman
V.Vox Sang. – 1971. – V. 20. – P. 271–273.
46 Nigg E.A., Bron C., Giradet M., Cherry R.J. Band 3-glycophorin A association in erythrocyte membranes demonstrated by combining protein diffusion measurements with antibody-induced cross-linking // Biochemistry. – 1980. – V. 19. – P. 1887–1193.
47 Oh S.S., Chisti A.H., Palek J., Liu S.-C. Erythrocyte membrane alterations in Plasmodium falciparum malaria sequestration // Curr. Opin. Hemat. – 1997. –V. 4. – P. 148–154.
48 Okubo Y., Yamaguchi H., Seno T. et al. The NFLD antigen in Japan // Hum. Hered. – 1988. –
38. – P. 122–124.
49 Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorfisms using the polymerase chain reaction // Genomics. – 1989. – V. 5. –
874–879.
193. Orlina A.R., DiMauro J., Unger P.J. Hemolytic disease of the newborn due to anti-Di b // Amer. J. Clin. Path. – 1979. – V. 71. – P. 713–714.
684
12 Paulitschke M., Nash G.B., Anstee D.J. et al. Perturbation of red blood cell membrane rigidity by extracellular ligands // Blood. – 1995. – V. 86. – P. 342–348.
13 Peters L.I., Shivdasani R.A., Liu S.C. et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton // Cell. – 1996. –
P. 86. – P. 917–927.
14 Poole J. Red cell antigens on band 3 and glycophorin A // Blood Rev. – 2000. – V. 14. –
B 31–43.
205. Poole J., Banks J., Kjeldsen-Kragh J. et al. Second example of MiV / MiV phenotype with anti-Wr b: a case study [Abstract] // Transfus. Med. – 1997. – V. 7 (Suppl. 1). – P. 27.
206. Poole J., Bruce L.J., Hallewell H. et al. Erythrocyte band 3 mutation Pro566→Ser gives rise to the BOW antigen and Pro561→Ala to a novel antigen KREP [Abstract] // Transfus. Med. – 1998. – V. 8(Suppl. 1). – P. 17.
207. Poole J., Hallewell H., Bruce L. et al. Identification of two new Jn(a + ) individuals and assignment of Jn a to erythrocyte band 3 [Abstract] // Transfusion. – 1997. – V. 37 (Suppl.). – 90S.
208. Popov M., Tam L.Y., Jing L., Reithmeier R.A.F. Mapping the ends of transmembrane segments in a polytopic membrane protein: scanning N-glycosylation mutagenesis of extracytosolic loops in the anion exchanger, band 3 // J. Biol. Chem. – 1997. – V. 272. – P. 18325–18332.
209. Race R.R., Sanger R. Blood Groups in Man. – 6-th ed. – Oxford: BSP, 1975. – 659 p.
210. Ranney H.M., Rosenberg G.H., Morrison M., Mueller T.J. Frequencies of band 3 variants of human red cell membranes in some different populations // Brit. J. Haemat. – 1990. – V. 75. –
P. 262–267.
211. Rearden A. Reactivity of monoclonal anti-Wr b with a synthetic peptide // Transfusion. – 1989. – V. 29. – P. 187.
212. Reid M.E., Lisowska E., Blanchard D. eds. Third International Workshop on Monoclonal Antibodies Against Human Red Blood Cell and Related Antigens // Transfus. Clin. Biol. – 1997. – V. 4. – P. 57–96 (9 papers).
213. Reid M.E., Lomas-Francis C. The Blood Group Antigen: FactsBook. – 2-nd ed. – London: Academic Press, 2004. – 561 p.
214. Ribeiro M.L., Alloisio N., Almeida H. et al. Severe hereditary spherocytosis and distal renal tubular acidosis associated with total absence of band 3 // Blood. – 2000. – V. 96. –
P. 1602–1604.
215. Riches R.A., Laycock C.M., Poole J. Anti-Di a causing HDN in an English family: non-linkage of Diego and Colton genes is demonstrated [Abstract] // 20-th Congr. Int. Soc. Blood Transfus., 1988. – P. 299.
216. Ridgwell K., Tanner M.J.A., Anstee D.J. The Wr b antigen in St a-positive and Dantu-positive human erythrocytes // J. Immunogenet. – 1984. – V. 11. – P. 365–370.
217. Ridgwell K., Tanner M.J.A., Anstee D.J. The Wr b antigen, a receptor for Plasmodium falciparum malaria, is located on a helical region of the major membrane sialoglycoprotein of human red blood cells // Biochem. J. – 1983. – V. 209. – P. 273–276.
218. Ring S.M., Green C.A., Swallow D.M., Tippett P. Production of a murine monoclonal antibody to the low incidence red cell antigen Wr a: characterization and comparison with human anti-Wr a // Vox Sang. –1994. – V. 67. – P. 222–225.
219. Ring S.M., Tippett P., Swallow D.M. Comparative immunochemical analysis of Wr a and Wr b red cell antigens // Vox Sang. – 1994. –V. 67. – P. 226–230.
220. Rouger P., Anstee D., Salmon C. eds. First International Workshop on Monoclonal Antibodies Against Human Red Blood Cell and Related Antigens // Rev. Franc. Transfus. Immunohemat. – 1988. – V. 31. – P. 261–364 (11 papers).
221. Rowe G.P., Hammond W. A new low-frequency antigen, Hg a (Hughes) // Vox Sang. – 1983. – V. 45. – P. 316–319.
685
26 Salhany J.M., Sloan R.L., Schopfer L.M. Characterization of the stilbenedisulphonate binding site on band 3 Memphis variant II (Pro-854→Leu) // Biochem. J. – 1996. – V. 317. – P. 509–514.
27 Schofield A.E., Martin P.G., Spiller D., Tanner M.J.A. The structure of the human red blood cell anion exchanger (EPB3, AE1, Band 3) gene // Blood. – 1994. – V. 84. – P. 2000–2012.
28 Simmons R.T. The apparent absence of the Diego (Di a) and Wright (Wr a) blood group antigens in Australian Aborigines and New Guineans // Vox Sang. – 1970. – V. 19. – P. 533–536.
29 Simmons R.T., Albrey J.A., Morgan J.A.G., Smith J.A. The Diego blood group: anti-Di a and the Di(a + ) blood group antigen found in Caucasians // Med. J. Aust. – 1968. – V. 1. – P. 406–407.
30 Smythe J.S., Spring F.A., Gardner B. et al. Monoclonal antibodies recognizing epitopes on the extracellular face and intracellular N-terminus of the human erythrocyte anion transporter (band 3) and their applications to the analysis of South East Asian ovalocytes // Blood. – 1995. – V. 85. – P. 2929–2936.
31 Southgate C.D., Chisti A.H., Mitchel B. et al. Targeted disruption on the murine erythroid band 3 gene results in spherocytosis and severe haemolytic anaemia despite a normal membrane skeleton // Nature Genet. – 1996. – V. 14. – P. 227–230.
32 Southscott M.J.G., Tanner M.J.A., Anstee D.J. The expression of human blood group during erythropoesis in a cell culture system // Blood. – 1999. – V. 93. – P. 4425–4435.
33 Spring F.A., Bruce L.J., Anstee D.J., Tanner M.J.A. Band 3 Memphis variant II: altered stilbene disulphonate binding is associated with the Diego (Di a) blood group antigen // Biochem. J. – 1992. – V. 288. – P. 713–716.
34 Tanner M.J.A. Molecular and cellular biology of the erythrocyte anion exchanger (AE1) // Semin. Hematol. – 1993. – V. 30. – P. 34–57.
35 Tanner M.J.A. The structure and function of band 3 (AE1): recent developments (review) // Mol. Mem. Biol. – 1997. – V. 14. – P. 155–165.
36 Tanner M.J.A., Martin P.G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence // Biochem. J. – 1988. – V. 256. – P. 703–712.
37 Tanphaichitr V.S., Sumboonnamonda A., Ideguchi H. et al. Novel AE1 mutations in the recessive distal renal tubular acidosis: loss-of-function is rescued by glycophorin A //
V. Clin. Invest. – 1998. – V. 102. – P. 2173–2179.
38 Tatarsky J., Stroup M., Levine P., Ernoenazy W.S. Another example of anti-Diego (Di a) // Vox Sang. – 1959. – V. 4. – P. 152–154.
39 Telen M.J., Chasis J.A. Relationship of the human erythrocyte Wr b antigen to an interaction between glycophorin A and band 3 // Blood. – 1990. – V. 76. – P. 842–848.
40 Thompson P.R., Childers D.M., Hatcher D.E. Anti-Di b: first and second examples // Vox Sang. – 1967. – V. 13. – P. 314–318.
41 Tills D., Kopec A.C., Tills R.E. The Distribution of Human Blood Groups and Other Polymorphisms. (Suppl. 1). – Oxford: University Press, 1983.
42 Uchikawa M., Shibata Y., Tohyama H. et al. A case of hemolytic disease if the newborn due to anti-Di b antibodies // Vox Sang. –1982. – V. 42. – P. 91–92.
43 Van Dort H.M., Moriyama R., Low P.S. Effect of band 3 subunit equilibrium on the kinetics and affinity of ankyrin binding to erythrocyte membrane vesicles // J. Biol. Chem. – 1998. –
V. 273 – P. 14819–14826.
24 van Loghem J.J., van der Hart M., Bok J., Brinkering P.C. Two further examples of the antibody anti-Wr a (Wright) // Vox Sang (old series). – 1955. – V. 5. – P. 130–134.
25 Wainwright S.D., Tanner M.J.A., Martin G.E.M. et al. Monoclonal antibodies to the membrane domain of the human erythrocyte anion transport protein. Localization of the C-terminus of the protein to the cytoplasmic side of the red cell membrane and distribution of the protein in some human tissues // Biochem. J. – 1989. – V. 258. – P. 211–220.
26 Walker M.F., Tippett P.A., Roper J.M. et al. Tests with some rare blood-group antibodies // Vox Sang. –1961. – V. 6. – P. 357.
686
249. Wallis J.P., Hedley G.P., Charlton D. et al. The incidence of anti-Wr a and Wr a antigen in blood donors and hospital patients // Transfus. Med. – 1996. – V. 6. – P. 361–364.
250. Wang L., Groves J.D., Mawby W.J., Tanner M.J.A. Complementation studies with co-expressed fragments of the human red cell anion transporter (band 3; AE1): the role of some exofacial loops in anion transport // J. Biol. Chem. – 1997. – V. 272. – P. 10631–10638.
251. Wiener A.S., Brancato G.J. Severe erythroblastosis fetalis caused by sensitization to a rare human agglutinogen // Amer. J. Hum. Genet. – 1953. – V. 5. – P. 350–355.
252. Won S.D., Shin H.S., Kim S.W. et al. Distribution of hereditary blood factors among Koreans residing in Seoul, Korea // Amer. J. Phys. Anthrop. – 1960. – V. 18. – P. 115–124.
253. Wren M.R., Issitt P.D. Evidence that Wr a and Wr b are antithetical // Transfusion. – 1988. – V. 28. – P. 113–118.
254. Yannoukakos D., Vasseur C., Driancourt C. et al. Human erythrocyte band 3 polymorphism (band 3 Memphis): characterization of the structural modification (Lys56→Glu) by protein chemistry methods // Blood. – 1991. – V. 78. – P. 1117–1120.
255. Yasuda H., Ohto H., Yamaguchi O. et al. Three episodes of delayed hemolytic transfusion reactions due to multiple red cell antibodies, anti-Di a, anti-Jk a and anti-E // Transfus. Sci. – 2000. – V. 23. – P. 107–112.
256. Young S. Vg a: a new low incidence red cell antigen // Vox Sang. – 1981. – V. 41. – P. 48–49.
257. Young S., Mallan M., Case J. et al. Further examples of the Wulfsberg antigen // Vox Sang. – 1980. – V. 38. – P. 213–215.
258. Zafar M., Reid M.E. Review: the Diego blood group system // Immunohematology. – 1993. – V. 9. – P. 35–40.
259. Zago-Novaretti M.C., Soares M.O.C., Dorlhias-Llacer P.E., Chamone D.A.F. Anti-Diego in multitransfused patients [Abstract] // Transfus. Sci. – 1992. – V. 110. – IH52.
260. Zelinski T., McManus K., Punter F. et al. A Gly565→Ala substitution in human erythrocyte band 3 accounts for the Wu blood group polymorphism // Transfusion. – 1998. – V. 38. – P. 745–748.
261. Zelinski T., Punter F., McManus K., Coghlan G. The ELO blood group polymorphism is located in the putative first extracellular loop of human erythrocyte band 3 // Vox Sang. – 1998. – V. 75. – P. 63–65.
262. Zelinski T., Rusnak A., McManus K., Coghlan G. Distinctive Swann blood group genotypes: molecular investigations // Vox Sang. – 2000. – V. 79. – P. 215–218.
263. Zupanska B., Brojer E., McIntosh J. et al. Correlation of monocyte-monolayer assay results, number of erythrocyte-bound IgG molecules, and IgG subclass composition in the study of red cell alloantibodies other than D // Vox Sang. – 1990. – V. 58. – P. 276–280.
687
Глава 13.
Система Cartwright
Система Cartwright (Картрайт) интересна тем, что носителями составляю-щих ее антигенов являются молекулы ацетилхолинэстеразы (АХЭ). Этот фер-мент участвует в передаче нервного импульса. Импульс передается на воспри-нимающие рецепторы очередного нейрона или мускульной клетки через синапс посредством образования ацетилхолина (АХ), который после проведения им-пульса разлагается АХЭ на холин и уксусную кислоту. Таким образом АХЭ вы-полняет роль биохимического реле, разделяющего нервные импульсы и одно-временно контролирующего состояние системы связи. Острая токсичность фос-форорганических соединений является прямым следствием того, что они явля-ются сильными ингибиторами АХЭ.
Ассоциационно-диссоциационная система АХ – АХЭ – АХ является осно-вой иннервации всего организма, включая периферическую (проводниковую) и центральную нервную систему. Благодаря ей нервные импульсы поступают во все ткани организма.
275. связи с этим можно высказать предположение о своеобразной иннервации циркулирующих клеток как в норме, так и в состоянии стресса. Стресс охваты-вает весь организм: и стационарные ткани, к которым подходят нервные окон-чания, и циркулирующие в кровотоке клетки, которых нервные окончания не достигают. Наличие АХЭ на эритроцитах, по-видимому, обеспечивает подведе-ние к ним нервного импульса (доведение до эритроцитов стрессового сигнала). Организм как целостная система таким образом доводит сигнал стресса и моби-лизационный импульс до всех тканей, стационарных и подвижных, обеспечивая ответную реакцию всего организма.
276. литературе имеются указания на то, что ген ACHE (acetylcholinester-ase) играет определенную роль в гемопоэзе. Аномалии хромосомы 7, где располагается этот ген, часто обнаруживают у пациентов с острым не-лимфоцитарным лейкозом и миелодисплазией, и наиболее частые хромо-сомные нарушения при этих состояниях происходят в 7q22, участке гена
ACHE (Kere и соавт., 1989).
Lapidot-Lifson и соавт. (1989) связывали аномальный мегакариоцитопо-эз с нарушениями в локусе ACHE, вызванными химиотерапией, облучением или отравлением фосфорорганическими соединениями. Факт влияния АХЭ на мегакариоцитопоэз подтверждают результаты экспериментов на мышах
(Patinkin и соавт., 1989).
688
Антигены Yt a и Yt b
Система Cartwright представлена двумя антигенами: Yt a и Yt b (табл. 13.1). Антитела анти-Yt a, открывающие часто встречающийся антиген Yt a, обнаруже-ны в 1956 г. Eaton и соавт. [12] при проведении пробы на индивидуальную со-вместимость. Через 8 лет Giles и Metaxas [16] описали антитетичный антиген Yt b, встречающийся относительно редко (с частотой ≈ 8 %).
| Таблица 13.1 | |||||
Антигены системы Yt
Обозначение
Характеристика
Нулевой фенотип Yt(a −b −), как и молчащий ген, передаваемый по наслед-ству, в системе Yt неизвестен.
Антигенные различия Yt a / Yt b обусловлены заменой гистидина на аспарагин в по-зиции 353 (рис. 13.1). Локусы YT и ACHE (рис. 13.2) не зависят от генов других групп крови. Они расположены на хромосоме 7 в позиции 7q22.1 (Reid, Lomas-Francis [40]).
171989–1991 гг. группа исследователей (Coghlan и соавт., Zelinski и соавт.) опублико-вала данные о возможной сцепленности локусов YT и KEL. Последний, как вскоре выяснилось, так же как и YT, расположен на хромосоме 7. Однако последующие ис-следования не подтвердили существование указанной сцепленности.
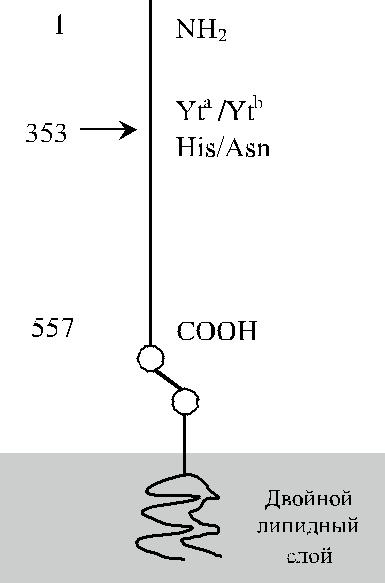
Рис. 13.1. Строение гликопро-
теина, несущего антигены Yt.
689
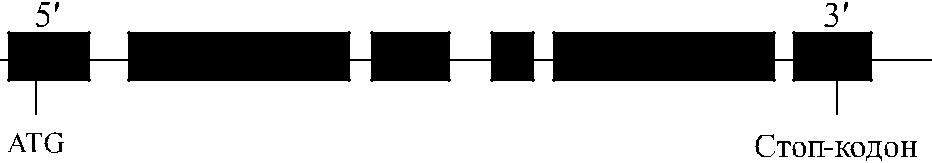
Рис. 13.2. Организация гена YT (ACHE).
Дата: 2019-02-24, просмотров: 891.