Давление насыщенного водяного пара при различных температурах
Поможем в ✍️ написании учебной работы
| °C | Р, Па | °С | Р, Па | °С | Р, Па |
| 0 | 610,4 | 14 | 1598,5 | 28 | 3780,0 |
| 1 | 657,2 | 15 | 1705,2 | 29 | 4005,0 |
| 2 | 705,2 | 16 | 1817,2 | 30 | 4242,2 |
| 3 | 757,3 | 17 | 1937,1 | 32 | 4753,0 |
| 4 | 813,3 | 18 | 2065,1 | 34 | 5318,0 |
| 5 | 871,9 | 19 | 2197,1 | 36 | 5940,0 |
| 6 | 934,6 | 20 | 2338,4 | 38 | 6623,0 |
| 7 | 1001,2 | 21 | 2486,4 | 40 | 7374,0 |
| 8 | 1073,2 | 22 | 2643,7 | 50 | 12334,0 |
| 9 | 1147,8 | 23 | 2809,1 | 60 | 19920,0 |
| 10 | 1227,9 | 24 | 2983,7 | 70 | 31160,0 |
| 11 | 1311,9 | 25 | 3167,7 | 80 | 43360,0 |
| 12 | 1402,5 | 26 | 3361,0 | 90 | 70100,0 |
| 13 | 1497,2 | 27 | 3565,0 | 100 | 101325,0 |
П р и л о ж е н и е 4
Произведения растворимости малорастворимых электролитов при 25 ° С
| Вещество | ПР | Вещество | ПР | Вещество | ПР |
| Ac(OH)3 | 2,10 ×10-19 | Cd3(AsO4)2 | 2,20 ×10-33 | PbI2 | 8,00 × 10-9 |
| Ag3AsO3 | 4,50 ×10-19 | CdCO3 | 1,00 ×10-12 | PbBr2 | 9,10 ×10-6 |
| AgBr | 6,00 ×10-13 | CdF2 | 6,44 ×10-3 | Pb3(PO4)2 | 7,90 ×10-43 |
| AgBrO3 | 5,38 ×10-5 | Cd(IO3)2 | 2,50 ×10-8 | PbC2O4 | 7,30 ×10-11 |
| AgCH3COO | 1,94 ×10-3 | CdS | 6,50 ×10-28 | PbMoO4 | 8,50 ×10-16 |
| AgCN | 5,97 ×10-17 | Cd(OH)2 | 7,20 ×10-15 | Pd(OH)2 | 1,20 ×10-29 |
| Ag2C2O4 | 5,40 ×10-12 | Cd(CN)2 | 1,00 ×10-8 | PdS | 7,50 ×10-48 |
| Ag2Cr2O7 | 2,00 ×10-7 | Cd3(PO4)2 | 2,53 ×10-33 | PtBr4 | 3,00 ×10-41 |
| Ag2Cr2O4 | 1,12 ×10-12 | Co3(AsO4)2 | 6,80 ×10-29 | Pt(OH)2 | 1,00 ×10-25 |
| Ag2O | 2,00 ×10-8 | CoCO3 | 2,80 ×10-10 | PtS | 1,20 ×10-61 |
| Ag2CO3 | 8,46 ×10-12 | Co3(PO4)2 | 2,05 ×10-35 | Sb2S3 | 2,20 ×10-90 |
| AgCl | 1,80 ×10-10 | CsClO4 | 3,95 ×10-3 | ScAsO4 | 1,90 ×10-27 |
| AgI | 1,10 ×10-16 | CsIO3 | 1,00 ×10-2 | Sc(OH)3 | 8,70 ×10-28 |
| AgIO3 | 3,17 ×10-8 | CsIO4 | 5,16 ×10-6 | Sn(OH)2 | 3,70 ×10-15 |
| Ag3PO4 | 1,80 ×10-18 | CsMnO4 | 9,10 ×10-5 | SnS | 3,00 ×10-28 |
| Ag2S | 5,70 ×10-51 | Ce(OH)3 | 6,40 ×10-22 | SnI2 | 8,30 ×10-6 |
| Ag2SO3 | 1,50 ×10-14 | CuC2O4 | 2,90 ×10-8 | SnS2 | 2,30 ×10-58 |
| Ag2SO4 | 1,20 ×10-5 | Cu(IO3)2 | 1,40 ×10-7 | Zn3(PO4)2 | 9,10 ×10-33 |
| AgSCN | 1,03 ×10-12 | Cu3(AsO4)2 | 7,60 ×10-36 | Zn(CN)2 | 2,60 ×10-13 |
| Ag2Se | 2,50 ×10-59 | CuCO3 | 2,36 ×10-10 | ZnCO3 | 6,00 ×10-11 |
| Ag2Te | 4,70 ×10-52 | Cu(OH)2 | 2,20 ×10-20 | Zn(OH)2 | 1,00 ×10-17 |
| Ag2SO4 | 7,70 ×10-5 | CuS | 6,00 ×10-36 | ZnS | 1,60 ×10-24 |
| Am(OH)3 | 2,70 ×10-20 | Fe(OH)2 | 1,00 ×10-15 | ZnC2O4 | 2,00 ×10-8 |
| Am(OH)4 | 1,00 ×10-56 | Fe(OH)3 | 3,80 ×10-38 | SrWO4 | 7,10 ×10-9 |
| AuBr | 5,00 ×10-17 | FeS | 3,40 ×10-17 | SrCO3 | 9,42 ×10-10 |
| AuCl | 1,80 ×10-12 | FePO4 | 1,10 ×10-26 | SrSO4 | 2,80 ×10-7 |
| AuI | 1,60 ×10-23 | FeSe | 1,80 ×10-20 | SrC2O4 | 5,60 ×10-8 |
| AuI3 | 1,00 ×10-46 | FeCO3 | 2,90 ×10-11 | Sr3(PO4)2 | 1,00 ×10-31 |
| Au2O3 | 8,50 ×10-46 | FeC2O4 | 2,10 ×10-7 | Sr(OH)2 | 3,20 ×10-4 |
| Al(OH)3 | 1,90 ×10-33 | MgCO3 | 7,90 ×10-6 | SrMoO4 | 1,60 ×10-7 |
| BaCO3 | 7,00 ×10-9 | MgS | 2,00 ×10-15 | SrCrO4 | 2,70 ×10-5 |
| BaC2O4 | 1,10 ×10-7 | MgC2O4 | 8,60 ×10-5 | SrF2 | 2,50 ×10-9 |
| Ba3(PO4)2 | 6,00 ×10-39 | MgF2 | 6,40 ×10-9 | Sr3(AsO4)2 | 1,30 ×10-18 |
| BaCrO4 | 1,60 ×10-10 | MgSO3 | 3,00 ×10-3 | SrSeO4 | 2,50 ×10-5 |
| BaF2 | 1,84 ×10-7 | MgSeO3 | 4,40 ×10-6 | TlBr | 4,30 ×10-6 |
| BaSO3 | 5,00 ×10-10 | Mg3(PO4)2 | 3,90 ×10-26 | Tl2CO3 | 4,00 ×10-3 |
| BaSO4 | 1,08 ×10-10 | MgWO4 | 3,60 ×10-7 | Tl2C2O4 | 2,00 ×10-4 |
| Ba(IO3)2 | 4,01 ×10-9 | Mg(IO3)2 | 3,00 ×10-13 | TlCl | 1,90 ×10-4 |
| BaMoO4 | 3,54 ×10-8 | Mg(OH)2 | 5,50 ×10-12 | Tl2CrO4 | 1,00 ×10-12 |
| Ba(NO3)2 | 4,64 ×10-3 | MnS | 2,50 ×10-10 | TlI | 6,60 ×10-8 |
| BaSeO4 | 3,40 ×10-8 | MnSO3 | 2,60 ×10-7 | Tl3PO4 | 6,70 ×10-8 |
| Ba(BrO3)2 | 2,43 ×10-4 | MnCO3 | 5,05 ×10-10 | Tl2SO4 | 1,50 ×10-4 |
| BiAsO4 | 4,43 ×10-10 | Mn(OH)2 | 2,30 ×10-13 | Tl2SeO4 | 1,00 ×10-4 |
| BiI3 | 7,71 ×10-19 | MnC2O4 | 8,60 ×10-5 | RbClO4 | 3,00 ×10-3 |
| BiClO | 3,40 ×10-36 | Ni(CN)2 | 3,00 ×10-23 | Pr(OH)3 | 3,39 ×10-24 |
| Bi(OH)3 | 3,00 ×10-36 | NiCO3 | 1,30 ×10-7 | Hg2Br2 | 7,90 ×10-23 |
| Bi2S3 | 8,90 ×10-10 | NiC2O4 | 4,00 ×10-10 | Hg2Cl2 | 1,50 ×10-18 |
| CaCO3 | 5,00 ×10-9 | Ni(OH)2 | 1,60 ×10-14 | Hg2I2 | 5,40 ×10-29 |
| Ca3(PO4)2 | 2,07 ×10-33 | NiS | 9,30 ×10-22 | Hg2SO4 | 6,20 ×10-7 |
| CaSO4 | 4,93 ×10-5 | NiSeO3 | 2,50 ×10-6 | HgS | 1,6×10-52 |
| CaC2O4 | 2,00 ×10-9 | PbCl2 | 2,0×10-5 | HgI2 | 2,8×10-29 |
| CaF2 | 4,00 ×10-11 | PbCrO4 | 1,8×10-14 | Hg2(SCN)2 | 3,20 ×10-20 |
| Ca(OH)2 | 5,02 ×10-6 | PbCO3 | 1,5×10-13 | HgBr2 | 6,20 ×10-20 |
| Ca3(AsO4)2 | 6,80 ×10-19 | PbS | 1,0×10-27 | Hg2C2O4 | 1,75 ×10-13 |
| CaWO4 | 1,60 ×10-9 | PbSO4 | 1,6×10-8 | Hg2CO3 | 3,60 ×10-17 |
П р и л о ж е н и е 5
Стандартные электродные потенциалы в водных растворах
| Электрод | Е0, В | Электрод | Е0, В |
| Li+/Li | – 3,045 | Tl +/Tl | – 0,336 |
| Rb+/Rb | – 2,925 | Co2+/Co | – 0,277 |
| K+/K | – 2,924 | Ni2+/Ni | – 0,257 |
| Cs+/Cs | – 2,923 | Mo3+/Mo | – 0,200 |
| Ba2+/Ba | – 2,912 | Sn2+/Sn | – 0,136 |
| Fr+/Fr | – 2,900 | Pb2+/Pb | – 0,126 |
| Sr2+/Sr | – 2,868 | Fe3+/Fe | – 0,036 |
| Ra2+/Ra | – 2,800 | 2H+/H2 | 0,000 |
| Ca2+/Ca | – 2,866 | Ge4+/Ge | +0,124 |
| La3+/La | – 2,379 | Ge2+/Ge | +0,240 |
| Y3+/Y | – 2,372 | Re3+/Re | +0,300 |
| Na+/Na | – 2,714 | Bi3+/Bi | + 0,308 |
| Mg2+/Mg | – 2,363 | Cu2+/Cu | + 0,342 |
| Be2+/Be | – 1,847 | Tc2+/Tc | +0,400 |
| Al3+/Al | – 1,662 | Ru2+/Ru | +0,455 |
| Ti 2+/Ti | – 1,628 | Cu+/Cu | + 0,521 |
| Zr4+/Zr | – 1,580 | Rh2+/Rh | +0,600 |
| Hf4+/Hf | – 1,550 | Tl 3+/Tl | + 0,741 |
| Ti 3+/Ti | – 1,370 | Rh3+/Rh | +0,758 |
| V2+/ V | – 1,186 | Po4+/Po | +0,760 |
| Mn 2+/ Mn | – 1,180 | Hg 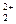 /Hg /Hg
| +0,797 |
| Cr 2+/Cr | – 0,913 | Ag+/Ag | + 0,799 |
| Zn2+/Zn | – 0,763 | Hg 2+/Hg | + 0,851 |
| Cr3+/Cr | – 0,744 | Pd2+/Pd | +0,951 |
| Ta 3+/Ta | – 0,600 | Ir3+/Ir | +1,156 |
| Ga3+/Ga | – 0,549 | Pt2+/Pt | + 1,190 |
| Fe2+/Fe | – 0,440 | Au3+/Au | + 1,498 |
| Cd2+/Cd | – 0,403 | Au+/Au | + 1,691 |
| In3+/In | – 0,338 |
П р и л о ж е н и е 6
Дата: 2019-02-25, просмотров: 348.