Select the correct scheme of the zinc-copper galvanic cell:
(-)Zn/ZnSO4//CuSO4/Cu(+)
Select the possible degrees of oxidation of the nitrogen atom in compounds:
-1, -2, -3, +1, +2, +3, +4, +5
Select the group of amphoteric oxides:
Show me the number of protons (p) and neutrons (n) in the isotope of element 60 Э145:
60p, 85n
Show me the number of electrons (e) and neutrons (n) in the isotope of element 50Э120 :
50e, 70n
Show me the number of protons (p) and electrons (e) in the isotope of element 80 Э120:
80e, 80p
Select impossible electron configuration:
1s3
Select a possible electron configuration:
3p5
Determine, where are p-blok elements?
В , С , N, O, F, Ne
Select the elements of III group, main subgroup:
B, Al, Ga, In, Tl
Select the elements of IV group, sub-group:
Cr, Mo, W
Select a strong electrolyte:
HCl
Select the electrolyte which dissociates in one step:
NaOH
State function is a property which depends upon:
Only initial and final states of system and not upon the path followed
The reaction of the salt with water, whereby the salt is dissociated and decomposed to form a weak electrolyte (weak acid or weak base) is called ...
Hydrolysis
This process is described by the equilibrium constant  :
:
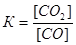
The electrode potential at an copper electrode, dipped in a 0,01М aqueous solution of copper sulfate at 25°C, is equal ....... 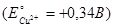 :
:
+0,281 В
The Nernst equation is described by:

The process of redox decomposition of an ionic compounds by passing electricity through molten compounds or aqueous solutions of compounds is called:
electrolysis
The chemical destruction of metal in fluids, electrolytes which are not is called:
Liquid corrosion
The greatest rate of corrosion will be observed between a pair of metal:
Ca-Cu
The most dangerous type of corrosion to the national economy is ...
galvanic corrosion
The greatest rate corrosion will be observed between a pair of metal:
Ni-Ag
The coordination number of the complex compound [Ni(NH3)6](NO3)2 is equal ...
6
The coordination number of the complex compound [Au(NH3)4]2(SO4)3 is equal ...
4
The electronic structure of the atom [Ar18] 3d6 4s2 is correspond to the element:
Fe
The electronic structure of the atom [Xe54] 4f14 5d6 6s2 is correspond to the element:
Os
The electronic structure of the atom 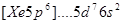 is correspond to the element:
is correspond to the element:
Ir
The process of flow of solvent molecules from pure solvent to the solution through semi permeable membrane called:
osmosis
Two different solutions having the same osmotic pressure at same temperature called:
Isotonic solutions
The properties of solutions which depends on the concentration of solute particles but irrespective of nature of solute particles called:
colligative
The titer of the nitric acid solution is 0.0082 g / ml. Acid mass in 500 ml of a solution is equal to (g):
4,1
The process of splitting of the electrolytes molecules into ions under the influence of polar molecules of solvent is called ...
dissociation
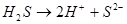 This process is described by the dissociation constant:
This process is described by the dissociation constant:
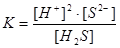
The velocity (rate) of chemical reaction is characterized by?
change of substances amounts for a time unit in unit of volume or unit of the area;
The velocity of direct reaction N2 + 3H2 = 2NH3 + Q increases if?
the concentration of nitrogen increases;
The velocity of chemical reaction between metal and sulfur does not depend on?
pressure
Temperature coefficient equals 2. The rate of reaction equals V1 at 20oC. At what temperature (in Celsius degrees oC) will the reaction rate change so V2 = 8V1?
30
The neutral moleculas or negative ions bound to the central metal or ion in the coordination sphere called:
ligand
Titer is defined as the number of:
Дата: 2018-12-28, просмотров: 909.