Anode is:
The electrode at which oxidation occurs
A system which can exchange only energy but not matter with the surrounding is known as:
Closed system
A solution contains 20.0g of glucose (C6H12O6, M=180g/mol) in 100 g of water. What is the freezing point of the solution ( Kf = 1.86°C / m )?
- 2.06°C
A device in which electric current is produced at the expense of spontaneous chemical reaction:
Both galvanic cell and voltaic cell
Among the following which is not a true statement for Faraday’s laws of electrolysis?
The weight of substance deposited is not directly proportional to the quantity of electricity passed
An aqueous solution of K2SO4 is electrolysed using platinum electrodes. The products at the anode and the cathode are:
O2, H2
A current of x A flowing for 10 min deposits 3g of the metal which is monovalent. The atomic mass of the metal is 50. Find the value of x?
9.65
An equivalent weight of oxidizer KMnO4 in a strong acidic medium is equal ... (g/mol):
31,6
An equivalent weight of oxidizer KMnO4 in a basic medium is equal ... (g/mol):
158
A system which can exchange energy and matter with the surrounding is known as:
Open system
A system which can not exchange by energy and matter with the surrounding is known as:
Isolated system
Applying the law of mass action to select the mathematical expression for the reaction rate 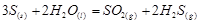 :
:
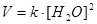
Applying the law of mass action to select the mathematical expression for the equilibrium constant of following reaction 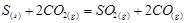 :
:
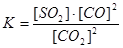
Base is a substance whose solution in water:
All of the above
Choose substances that form aqueous solution with acidic medium (pH<7):
CuSO4
Calculate the equivalent weight (g/mol) of the oxidant in the reaction: 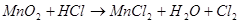
43,5
Calculate the equivalent weight (g/mol) of the reducing agent in the reaction: 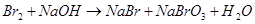
16
Calculate the electrode potential at an gold electrode, dipped in a 0,001М aqueous solution of gold salt at 25°C 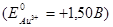 :
:
+1,441 В
Calculate the percent concentration of the solution obtained by dissolving 50g of alkali in 400g water (%):
11,1
Calculate the boiling point of an aqueous solution containing 18g of glucose (C6H12O6) in 100g of water. (Elevation constant for water EH2O = 0,52° Celsius)
100,52 ° Celsius
Compounds in which a central metal atom or ion is linked to a number of ions or neutral molecules by coordinate bonds or which contain complex ions is called:
Coordination compounds
Colloidal system where a liquid is dispersed in another liquid is called:
Emulsion
Colloidal system where a solid particals is dispersed in another liquid is called:
Suspension
Calculate the normality of a sulfuric acid (H2SO4) solution with a density of 1,2 g/mol and a percent concentration 30%:
7,34N
Copper (II) nitrate and sodium hydroxide solutions react in a test tube as shown below: Cu(NO3)2 + 2NaOH = Cu(OH)2 + 2NaNO3 If nitric acid is added to the test tube, the amount of solid precipitate decreases. The best explanation for this is that the acid:
Дата: 2018-12-28, просмотров: 823.