C6H6 + Br2 = C6H5Br + HBr Which of the following changes will cause an increase in the rate of the above reaction?
increasing the concentration of Br2
Cathode is:
The electrode at which reduction occurs
Chemical kinetics is the branch of chemistry which deals with the study of:
All of these
Calculate the standard cell potential if the standard reduction potential of Cu and Zn are + 0.34V and -0.76V respectively. The cell is shown below Zn / ZnSO4 // CuSO4 / Cu.
V
Ca and Cu metals when coupled together gives maximum emf for a voltaic cell because:
It shows greater difference in standard reduction potential values
During electrochemical corrosion of lead pipes in acidic soil, here takes place the following chemical process:
Pb-2e→Pb2+ , 2H+ +2e→H2
Determine the standard EMF of the cell: 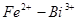
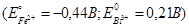
+0,65 В
Determine the standard EMF of the cell 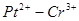 :
: 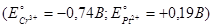
+0,93 В
Determine the standard EMF of the cell 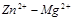 :
: 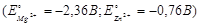
+1,60 В
Determine, where are p-blok elements?
В , С , N, O, F, Ne
Degree of ionization 'a' may be defined as ...
a fraction of total number of molecules of an electrolyte which dissociate into ions
Degree of dissociation is greater then 30% for following electrolytes:
HCl, NaOH
Degree of dissociation is lower then 3% for next electrolytes:
H2S, H2CO3
Degree of dissociation is nearly equal to 1 (or 100%) for:
Strong acids and strong bases
In the electrochemical series metals are arranged ...
in the order of increasing the electrode potential
For an aqueous solution with pH=10, 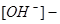 hydroxide ions concentration will be equal to.... (mol/L):
hydroxide ions concentration will be equal to.... (mol/L):
10-4
For 0,01М sodium hydroxide aqueous solution (NaOH) its рH value will be equal:
12
For an aqueous solution with pOH=5, hydrogen (H+) ions concentration will be equal to.... (mol/L):
10-9
From a mixture of cations in the solution are recovered in the first place:
Metals with the greatest value of the electrode potential
"For any solution, the partial vapour pressure of each volatile component in the solution is directly proportional to its mole fraction in the solution"
Who is the author of this law?
Raoult‘ s 2nd law: 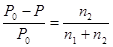
For chemical process FeO(s) + H2(g) = Fe(s) + H2O point out, in how many times decreases the velocity of direct reaction if concentration of hydrogen decreases in 3 times?
in 3 times
For which of the following classes of salts are all of its compounds soluble in water?
Nitrates
For a chemical reaction it is usually found that the reaction rate is faster at higher temperature. The rate increases because:
More reactants collide with energy equal to or greater than the activation energy
For the reaction at equilibrium: 2 NO(g) + O2(g) = 2 NO2 (g) which change will increase the amount of NO2(g)?
Add NO gas
Find the molarity of 0.585g NaCl present in 500 ml of solution
M
Formation of …………. takes place when Zn dissolves in an excess of NaOH.
Na2ZnO2
For the following reduction reaction how many coulombs are required? 1 mol of Cu2+ to Cu:
C
For the following reaction 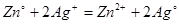 . Calculate the standard EMF of the cell. Given
. Calculate the standard EMF of the cell. Given 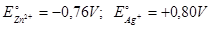
+1.56 volt
Find the valency of the metal whose atomic weight is 96 and 0.3605g of a metal is deposited on the electrode by passing 1.2 ampere current for 15 minutes:
3
Four alkali metals K, L, M, N and P are having standard electrode potentials as -4.05, -2.66, -0.50, 0.70 V and 0.25V respectively. Which one of the following is most reducing?
K
Galvanization of iron sheets is done by:
Zn plating
How many degrees the temperature of the system should be increased by increasing the reaction rate by 81 times, if the temperature coefficient is equal to γ = 3?
400 С
How many times does the chemical reaction rate change at the temperature rise from 25oC to 75oC if the temperature coefficient is equal 3?
increases in 243 times
How many times does the chemical reaction rate change at the temperature fall from 40oC if the temperature coefficient 4?
decreases in 256 times
Heterogeneous reaction occurs between:
magnesium and oxygen
How many grams of salt is necessary to prepare 0,1N 2L of 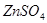 solution?
solution?
16,10
How many grams of glucose (C6H12O6) is dissolved in 500 g of water, if this solution has molality is equal 0,2 mol/kg ?
18 g
How many anions in the total amount are formed by the dissociation of the following electrolytes: 3 moles potassium carbonate (K2CO3) and 1 mole aluminium sulfate (Al2(SO4)3) ?
6
How many cations and anions in the total amount are formed by the dissociation of the next electrolytes: 2 moles sodium sulfide (Na2S) and 1 mole zinc nitrate (Zn(NO3)2)?
9
How many cations in the total amount are formed by the dissociation of the following electrolytes: 2 moles ammonium nitrate (NH4NO3) and 1 mole calcium chloride (CaCl2)?
3
How will affect 3 times increase of the pressure on the velocity of given reaction MgCO3 = MgO + CO2?
velocity doesn't change
How many times does the chemical reaction rate decrease at the temperature fall from 20oC to 0oC if the temperature coefficient is equal to 2?
in 4 times
How many electrons can occupy an s orbital?
two
H2O2, hydrogen peroxide, naturally breaks down into H2O and O2 over time. MnO2, manganese dioxide, can be used to lower the energy of activation needed for this reaction to take place and, thus, increase the rate of reaction. What type of substance is MnO2 ?
A catalyst
Hess’s law of constant heat summation states that:
Δ H is a state function
How many molecules are contained in the 5 liters of oxygen?
1,34*1023
In the electrochemical series metals are arranged ...
in the order of increasing the electrode potential
In how many times increases the velocity of elementary reaction 2A + B = 2C, if concentration of A decreases in 2 times?
decreases in 4 times
In which case the 2-time increase of the pressure doesn't influence the reaction rate?
Fe + CuSO4 = Cu + FeSO4
If temperature increases from 20oC to 50oC the velocity of reaction increases in 8 times. What the temperature coefficient is equal?
2
Indicate the oxidation state central metal atom and coordination number of complex ion [FeF6]3−
Oxidation state of Fe = +3; Coordination number = 6
In a coordination sphere, the atom/ion (usually metal) to which a fixed number of ions/moleculas/groups are bound in a definite geometrical arrangement around it, is called:
Central atom
In the periodic table, which of the following identifies a horizontal row?
period
In which of the following compounds is the bonding most covalent?
F2
In the common dry cell, the zinc atoms are:
Oxidized at the anode
I will be describing a chemical element. Try to identify it with the fewest number of clues. This chemical element is a silver-white metal, with bluish tinge, capableof taking a high polish. It is element occurs bundantly in all ordinary rocks, except limestone and sandstone; is third in abundance of the elements in the earth's crust and used in aviation and to make drink cans.The atomic number of this element is 13. Name it element:
Al
Intensive property of system is one whose:
Дата: 2018-12-28, просмотров: 896.