If the solvent boils at a temperature T1 and the solution at a temperature T2 , then the elevation of boiling point is given by:
T2 - T1
In the complex [Fe(H2O)5 (NO+)]SO4. The oxidation state of iron is:
+1
In which among the following are the noble metals soluble?
Aqua regia
In a redox reaction if the emf is positive then the reaction is:
Spontaneous
If the electrode potentials of both the electrodes become equal in magnitude but opposite in sign then:
An electrochemical cell stops working
In an electrochemical galvanic cell:
Chemical energy changes into electrical energy
In an electrolytic cell:
Electrical energy changes into chemical energy
In high energy density batteries Lithium is used as an electrode because:
It has high negative reduction potential
Mendeleev-Claperon’s eqution for gases is:
pV = nRT
Metal corrosion is ....
A spontaneous thermodynamic destruction metal as a result of exposure to chemical and electrochemical environment
Molarity is defined as the number of:
Moles of solute per liter of solution
Normal concentration of sulfuric acid (H2SO4) with titre of 0,0049 g / ml is equal:
0,10 н
Name the person who developed a table of elements which revealed regularities in elemental properties in 1869?
Dmitri Mendeleev
Normality is defined as the number of:
Equivalent weights of solute per liter of solution
NaCl in solid form does not conduct electricity because:
It does not ionize
0,5 moles of sugar (C12H22O11) dissolved in 500 g of water. What is the freezing point of this solution (КН2О=1,86˚)?
-1,86˚ С
Oxidation state of fluorine atom is equal:
0; -1
Ostwald law described by equation:
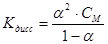
Of four different laboratory solutions, the solution with the highest acidity has a pH of:
2
Pure substances are conductors of the second type with an ionic conductivity and that dissociate into ions when dissolved in water or when electricity pass through its molten, and conduct electricity ade called ...
electrolytes
Redox reactions in which the both oxidizing and reducing agents are composed into one molecule is called:
Intramolecular
Select a typical oxidizing agent:
KMnO4
Select a typical reducing agent:
С ompound of iron (III) Fe3+
Select the oxidation process:
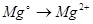
Select the oxidation process:
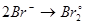
Select the reduction process:
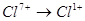
Select the reduction process:
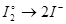
Select the definition of Faraday's 1st law:
The mass of a substance produced at an electrode during electrolysis is proportional to the number of moles of electrons (the quantity of electricity) transferred at that electrode
Select cause of electrochemical corrosion:
Дата: 2018-12-28, просмотров: 822.