Disproportionation
An equivalent weight of oxidizer KMnO4 in a water neutral medium is equal ... (g/mol):
52,6
Applying the law of mass action to select the mathematical expression for the reaction rate 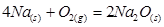 :
:
V = k [O2]
A solution containing 12.5 g of non-electrolyte substance in 175 g of water gave a boiling point elevation of 0.70. Calculate the molar mass of the substance. (Elevation constant for water EH2O = 0,52° Celsius)
53,06 g/mol
A potassium permanganate KMnO4 is a strong oxidizing agent in ................ medium
acidic
A potassium dichromate K2Cr2O7 acts as an oxidizing agent in ..................... medium
acidic
A concentration sodium phosphate solution is 17,0% (Na3PO4). The density of the solution is 0,900 g/mol. What is the molarity of the solution?
0,93 %
A solution with a pH of 12 is:
Strongly basic
All alloys contain this element if they are amalga. The element is:
Mercury
A double replacement reaction is likely to occur when :
Two ionic compounds are dissolved in water
A catalyst can speed up the rate of a given chemical reaction by:
Lowering the activation energy required for the reaction to occur
Anode is:
The electrode at which oxidation occurs
A system which can exchange only energy but not matter with the surrounding is known as:
Closed system
A solution contains 20.0g of glucose (C6H12O6, M=180g/mol) in 100 g of water. What is the freezing point of the solution ( Kf = 1.86°C / m )?
- 2.06°C
A device in which electric current is produced at the expense of spontaneous chemical reaction:
Both galvanic cell and voltaic cell
Among the following which is not a true statement for Faraday’s laws of electrolysis?
The weight of substance deposited is not directly proportional to the quantity of electricity passed
An aqueous solution of K2SO4 is electrolysed using platinum electrodes. The products at the anode and the cathode are:
O2, H2
A current of x A flowing for 10 min deposits 3g of the metal which is monovalent. The atomic mass of the metal is 50. Find the value of x?
9.65
An equivalent weight of oxidizer KMnO4 in a strong acidic medium is equal ... (g/mol):
31,6
An equivalent weight of oxidizer KMnO4 in a basic medium is equal ... (g/mol):
158
A system which can exchange energy and matter with the surrounding is known as:
Open system
A system which can not exchange by energy and matter with the surrounding is known as:
Isolated system
Applying the law of mass action to select the mathematical expression for the reaction rate 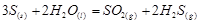 :
:
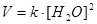
Applying the law of mass action to select the mathematical expression for the equilibrium constant of following reaction 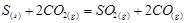 :
:
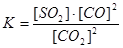
Base is a substance whose solution in water:
All of the above
Choose substances that form aqueous solution with acidic medium (pH<7):
CuSO4
Calculate the equivalent weight (g/mol) of the oxidant in the reaction: 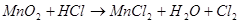
43,5
Calculate the equivalent weight (g/mol) of the reducing agent in the reaction: 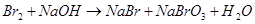
16
Calculate the electrode potential at an gold electrode, dipped in a 0,001М aqueous solution of gold salt at 25°C 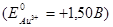 :
:
+1,441 В
Calculate the percent concentration of the solution obtained by dissolving 50g of alkali in 400g water (%):
11,1
Calculate the boiling point of an aqueous solution containing 18g of glucose (C6H12O6) in 100g of water. (Elevation constant for water EH2O = 0,52° Celsius)
100,52 ° Celsius
Compounds in which a central metal atom or ion is linked to a number of ions or neutral molecules by coordinate bonds or which contain complex ions is called:
Coordination compounds
Colloidal system where a liquid is dispersed in another liquid is called:
Emulsion
Colloidal system where a solid particals is dispersed in another liquid is called:
Suspension
Calculate the normality of a sulfuric acid (H2SO4) solution with a density of 1,2 g/mol and a percent concentration 30%:
7,34N
Copper (II) nitrate and sodium hydroxide solutions react in a test tube as shown below: Cu(NO3)2 + 2NaOH = Cu(OH)2 + 2NaNO3 If nitric acid is added to the test tube, the amount of solid precipitate decreases. The best explanation for this is that the acid:
All of these
Calculate the standard cell potential if the standard reduction potential of Cu and Zn are + 0.34V and -0.76V respectively. The cell is shown below Zn / ZnSO4 // CuSO4 / Cu.
V
Ca and Cu metals when coupled together gives maximum emf for a voltaic cell because:
Nitrates
For a chemical reaction it is usually found that the reaction rate is faster at higher temperature. The rate increases because:
Add NO gas
Find the molarity of 0.585g NaCl present in 500 ml of solution
M
Formation of …………. takes place when Zn dissolves in an excess of NaOH.
Na2ZnO2
For the following reduction reaction how many coulombs are required? 1 mol of Cu2+ to Cu:
C
For the following reaction 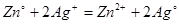 . Calculate the standard EMF of the cell. Given
. Calculate the standard EMF of the cell. Given 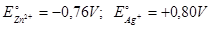
+1.56 volt
Find the valency of the metal whose atomic weight is 96 and 0.3605g of a metal is deposited on the electrode by passing 1.2 ampere current for 15 minutes:
3
Four alkali metals K, L, M, N and P are having standard electrode potentials as -4.05, -2.66, -0.50, 0.70 V and 0.25V respectively. Which one of the following is most reducing?
K
Galvanization of iron sheets is done by:
Zn plating
How many degrees the temperature of the system should be increased by increasing the reaction rate by 81 times, if the temperature coefficient is equal to γ = 3?
400 С
How many times does the chemical reaction rate change at the temperature rise from 25oC to 75oC if the temperature coefficient is equal 3?
increases in 243 times
How many times does the chemical reaction rate change at the temperature fall from 40oC if the temperature coefficient 4?
decreases in 256 times
Heterogeneous reaction occurs between:
magnesium and oxygen
How many grams of salt is necessary to prepare 0,1N 2L of 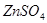 solution?
solution?
16,10
How many grams of glucose (C6H12O6) is dissolved in 500 g of water, if this solution has molality is equal 0,2 mol/kg ?
18 g
How many anions in the total amount are formed by the dissociation of the following electrolytes: 3 moles potassium carbonate (K2CO3) and 1 mole aluminium sulfate (Al2(SO4)3) ?
6
How many cations and anions in the total amount are formed by the dissociation of the next electrolytes: 2 moles sodium sulfide (Na2S) and 1 mole zinc nitrate (Zn(NO3)2)?
9
How many cations in the total amount are formed by the dissociation of the following electrolytes: 2 moles ammonium nitrate (NH4NO3) and 1 mole calcium chloride (CaCl2)?
3
How will affect 3 times increase of the pressure on the velocity of given reaction MgCO3 = MgO + CO2?
velocity doesn't change
How many times does the chemical reaction rate decrease at the temperature fall from 20oC to 0oC if the temperature coefficient is equal to 2?
in 4 times
How many electrons can occupy an s orbital?
two
H2O2, hydrogen peroxide, naturally breaks down into H2O and O2 over time. MnO2, manganese dioxide, can be used to lower the energy of activation needed for this reaction to take place and, thus, increase the rate of reaction. What type of substance is MnO2 ?
A catalyst
Hess’s law of constant heat summation states that:
Δ H is a state function
How many molecules are contained in the 5 liters of oxygen?
1,34*1023
In the electrochemical series metals are arranged ...
in the order of increasing the electrode potential
In how many times increases the velocity of elementary reaction 2A + B = 2C, if concentration of A decreases in 2 times?
decreases in 4 times
In which case the 2-time increase of the pressure doesn't influence the reaction rate?
Fe + CuSO4 = Cu + FeSO4
If temperature increases from 20oC to 50oC the velocity of reaction increases in 8 times. What the temperature coefficient is equal?
2
Indicate the oxidation state central metal atom and coordination number of complex ion [FeF6]3−
Oxidation state of Fe = +3; Coordination number = 6
In a coordination sphere, the atom/ion (usually metal) to which a fixed number of ions/moleculas/groups are bound in a definite geometrical arrangement around it, is called:
Central atom
In the periodic table, which of the following identifies a horizontal row?
period
In which of the following compounds is the bonding most covalent?
F2
In the common dry cell, the zinc atoms are:
Oxidized at the anode
I will be describing a chemical element. Try to identify it with the fewest number of clues. This chemical element is a silver-white metal, with bluish tinge, capableof taking a high polish. It is element occurs bundantly in all ordinary rocks, except limestone and sandstone; is third in abundance of the elements in the earth's crust and used in aviation and to make drink cans.The atomic number of this element is 13. Name it element:
Al
Intensive property of system is one whose:
Aqua regia
In a redox reaction if the emf is positive then the reaction is:
Spontaneous
If the electrode potentials of both the electrodes become equal in magnitude but opposite in sign then:
Dmitri Mendeleev
Normality is defined as the number of:
It does not ionize
0,5 moles of sugar (C12H22O11) dissolved in 500 g of water. What is the freezing point of this solution (КН2О=1,86˚)?
-1,86˚ С
Oxidation state of fluorine atom is equal:
0; -1
Ostwald law described by equation:
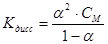
Of four different laboratory solutions, the solution with the highest acidity has a pH of:
2
Pure substances are conductors of the second type with an ionic conductivity and that dissociate into ions when dissolved in water or when electricity pass through its molten, and conduct electricity ade called ...
electrolytes
Redox reactions in which the both oxidizing and reducing agents are composed into one molecule is called:
Intramolecular
Select a typical oxidizing agent:
KMnO4
Select a typical reducing agent:
С ompound of iron (III) Fe3+
Select the oxidation process:
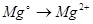
Select the oxidation process:
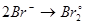
Select the reduction process:
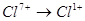
Select the reduction process:
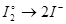
Select the definition of Faraday's 1st law:
The mass of a substance produced at an electrode during electrolysis is proportional to the number of moles of electrons (the quantity of electricity) transferred at that electrode
Select cause of electrochemical corrosion:
Ca-Cu
The most dangerous type of corrosion to the national economy is ...
galvanic corrosion
The greatest rate corrosion will be observed between a pair of metal:
Ni-Ag
The coordination number of the complex compound [Ni(NH3)6](NO3)2 is equal ...
6
The coordination number of the complex compound [Au(NH3)4]2(SO4)3 is equal ...
4
The electronic structure of the atom [Ar18] 3d6 4s2 is correspond to the element:
Fe
The electronic structure of the atom [Xe54] 4f14 5d6 6s2 is correspond to the element:
Os
The electronic structure of the atom 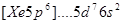 is correspond to the element:
is correspond to the element:
Ir
The process of flow of solvent molecules from pure solvent to the solution through semi permeable membrane called:
osmosis
Two different solutions having the same osmotic pressure at same temperature called:
Isotonic solutions
The properties of solutions which depends on the concentration of solute particles but irrespective of nature of solute particles called:
colligative
The titer of the nitric acid solution is 0.0082 g / ml. Acid mass in 500 ml of a solution is equal to (g):
4,1
The process of splitting of the electrolytes molecules into ions under the influence of polar molecules of solvent is called ...
dissociation
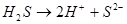 This process is described by the dissociation constant:
This process is described by the dissociation constant:
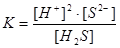
The velocity (rate) of chemical reaction is characterized by?
change of substances amounts for a time unit in unit of volume or unit of the area;
The velocity of direct reaction N2 + 3H2 = 2NH3 + Q increases if?
the concentration of nitrogen increases;
The velocity of chemical reaction between metal and sulfur does not depend on?
pressure
Temperature coefficient equals 2. The rate of reaction equals V1 at 20oC. At what temperature (in Celsius degrees oC) will the reaction rate change so V2 = 8V1?
30
The neutral moleculas or negative ions bound to the central metal or ion in the coordination sphere called:
ligand
Titer is defined as the number of:
Alkali metals
The elements in group IIA of the periodic table are called:
Alkaline earths
The bond in hydrogen chloride is which of the following types? Is it:
Polar covalent
The bond in Fluorine is which of the following types? Is it:
Nonpolar covalent
The process of conversion of solid into vapour directly is known as:
Sublimation
The first law of thermodynamics is also known as:
All of these
The rate of reaction depends upon the molar concentration of reactants which:
Activation energy
The ionic reactions are very fast because:
Solid
The value of Ebullioscopic constant or boiling point elevation constant depends on:
Nature of solvent
The value of Vant Hoff factor (i) = 2 is for:
Sodium chloride
The value of Vant Hoff factor (i) = 1 is for:
Glucose
The molarity of 4.6 N H2SO4 solution is:
M
The ratio of elevation in boiling point to molality of solution is known as:
Molal elevation constant
The quantity of electricity required to liberate or deposit 1 gram equivalent of a substance from its solution during electrolysis is known as:
Faraday
The constitute of a Danlell cell is:
Zn - Cu cell
The chemical equivalent of the metal is 19.3. When 3A of electric current is passed for 50 min. What will be the amount of metal deposited from an electrolyte?
G
The dissociation and ionization are partically same as both results in giving …………… of the substance.
Pressure
This type, of corrosion occurs due to direct chemical attack of environment or atmospheric gases like oxygen halogens, hydrogen sulphide, sulphurdioxide, nitrogen or anhydrous inorganic liquid with metal surfaces in immediate proximity. What is the corrosion type?
Chemical corrosion
This type of corrosion occurs when the metal comes in contact with a conducting liquid or when two dissimilar metals are immersed or dipped partly in a solution. There is the formation of a galvanic cell on the surface of metals. Some parts of the metal surface act as anode and rest act as cathode. Water must be present to serve as a medium for the transport of ions. What is the corrosion type?
Electrochemical corrosion
The reaction of the salt with water, whereby the salt is dissociated and decomposed to form a weak electrolyte is called ……………
Hydrolysis
Using IUPAC norms write the formula for the following: Hexaamminecobalt(III) sulphate
[Co(NH3)6]2 (SO4)Use the activity series given to you to determine which of the following reactions will NOT take place:Cu + HCl -->
Unit of molarity is:mol / litre
Vant-Hoff rule is described by following equation:
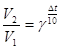
Variation of chemical elements that have the same number of protons but different number of total neutrons is known as:
Isotopes
What is the equivalent weight of the compound Ca3(PO4)2 (g-eq/mol)? Name it
51,6 (calcium phosphate)
What is the equivalent weight of the compound Fe(OH)3 (g-eq/mol)? Name it
35,6 (trihydroxide iron)
What is the equivalent weight of the compound PbO2 (g-eq/mol)? Name it4)
59,75 (lead dioxide)
What is the equivalent weight of the compound H3PO4 (g-eq/mol)? Name it4
32,6
What is the equivalent weight of the compound Al2(SO4)3 (g-eq/mol)? Name it
57 (dialuminium trisulfate)
What is the equivalent weight of the compound Na2SiO3 (g-eq/mol)? Name it
61 (disodium silicate)
What is the equivalent weight of the compound MnO3 (g-eq/mol)? Name it
17,16 (manganese trioxide)
What is the equivalent weight of the compound NH4ОН (g-eq/mol)? Name it
35 (ammonium hydroxide)
What is the eqiuvalen weight of compound Н2SO4 (g-eq/mol)? Name it
49 (sulfuric acid)
Write down the law of mass action for the given direct reaction: 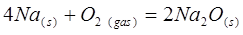
V = k [O2]
"When a system at equilibrium is subjected to change in concentration, temperature, volume, or pressure, then the system readjusts itself to (partially) counteract the effect of the applied change and a new equilibrium is established" This definition conforms to the law:
Le Châtelier's principle
Write down the law of mass action for the given direct reaction: 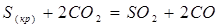
V= k[C O2 ]2
When the pressure increases and the concentration of hydrogen in the system: 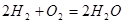 , the equilibrim will be shifts to ............
, the equilibrim will be shifts to ............
to right
When the temperature and the concentration of ammonium increases in the system: 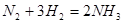 (∆H<O) , the equilibrim will be shifts to ............
(∆H<O) , the equilibrim will be shifts to ............
To left
What is the osmotic pressure, in kilopascal of 0,1M benzene 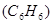 solution at
solution at 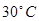 Celsius?
Celsius?
251,9kPa
What is the osmotic pressure, in atmospheres of 2M ethanol solution at 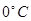 Celsius (R=0,082 L atm/mol K)?
Celsius (R=0,082 L atm/mol K)?
44,772 atm
What is the boiling point elevation of 2m formalin aqueous solution (ЕН2О= 0,52˚C)?
1,04˚ С
What is the freezing point of 1m glycerol 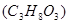 aqueous solution (КН2О=1,86˚)?
aqueous solution (КН2О=1,86˚)?
-1,86˚ С
What is the heat of reaction: 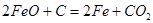 :
:
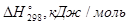 -264,45 0 0 -393,5
-264,45 0 0 -393,5
-135,4 кДж / моль
What is the enthalpy change of the reaction: 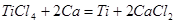
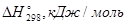 +763 0 0 -786
+763 0 0 -786
-2335,0 кДж / моль
What is the entropy change of the reaction (J/mol*K): 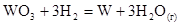
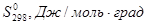 6 131 33 131
6 131 33 131
+27,0 Дж / моль * град
What is the change in Gibbs energy of reaction:
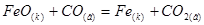
D G298 kL/mol -244,30 -137,15 0 -393,37
-11,92 кДж / моль
Which salt is hydrolyzed by cation?
ZnCl2
What salt is not hydrolyzed?
BaCl2
What salt aqueous solution will have alkaline medium (pH>7)?
К2S
Which salt is hydrolyzed by anion?
CH3COONa
Where does the acid base indicator - litmus gain of red colour?
CuCl2
Where does the acid base indicator phenolphthalein gain of crimson (purple) colour?
Na2 С O3
Weak basis and weak acid that form salt XA, if such ionic - molecular equation X+ + A- + H2O = XOH + HA correspond to its hydroysis. What salt is it?
NH4NO2
What is the pH value of the solution, with a content of [OH-] = 10-3 mol /L?
11
What is the pOH of the solution, with a content of [H+] = 10-4 mol /L?
10
What kind of water is obtained by passing the natural water through a system of cation and anion exchangers:
distilled water
What is the total hardness of water (mmol/L), if its 1 liter contains 40,04 mg of calcium (Ca2+) ions and 48.24 mg magnusium (Mg2+) ions?
6
Water hardness is characterized by the presence of dissolved salts of calcium and magnesium bicarbonates, called...
temparary hardness
What is the temporary hardness of water, if for titration of 100 ml of water was consumed 5.65 mL of 0.02 N hydrochloric acid solution (mmol / L)? 1,13
What is the total hardness of water, if for titration of 50 ml of water was consumed 2.5 ml of 0.025 N solution of EDTA (mmol / L)?
1,25
What is the electrode potential of a half cell consisting of zinc electrode in 0,01М ZnCl2 aqueous solution at 25°C 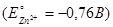 :
:
-0,819 В
What will be the electrode potential at an aluminium electrode, dipped in a 0,001М AlCl3 aqueous solution at 25°C 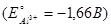 :
:
-1,719 В
What the metal cations will be recovered from their salt solutions by iron?
Sn, Pb
What the metal cations will be recovered from their salt solutions by magnesium?
Zn, Mn
What metals will react with diluted acids, as HCl and H2SO4, and produce hydrogen gas?
Al, Zn
What metals will not react with diluted acids?
Pt, Au
Which of the following metal ions will discharge first at electrode?
Ag+ (E ° = +0,80V)
Which of the following metal ions, as Al3+, Na+, Mg2+, Ag+, will discharge first at electrode during molten electrolysis?
Ag+, Al3+, Mg2+, Na+,
Which metals will serve as the anode for lead?
Cr, Ni
Which metal will serve as the cathode for iron?
Cu, Ni
Which metals acts as a protector for the iron and steel constructions?
Zn, Mg
When the value of the electrode potential of the metal increases in the electrochemical series, we observe that .....
reducing ability of the metal cation increasesWhat is the oxidation state of the central metal atom in the complex [Cu(NH3)4]SO4 :
+2
What is the oxidation state of the central metal atom in the complex Na2[Co(NH3)2Cl4] :
+2
What is a name this compound NaH2PO4?
sodium dihydrogenphosphate
Which group contains only the salts?
KBr, Na2SO4, FeCl3, CaCl2
What is the oxidation state (OS) of the central metal atom and the coordination number (CN) in the complex [Co(NH3)5 Cl ]Cl ? Name it
OS = +2, CN = 6. Pentaaminochloridocobalt (II) chloride
What is the oxidation state (OS) of the central metal atom and the coordination number (CN) in the complex Na3[Co(H2O)2Cl2PO4] :
OS =+2; CN = 5
Who is the author of this law?
Raoult‘ s 2nd law: 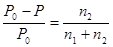
What is the molarity of 400 ml of a solution wherein dissolved 20g barium chloride BaCl2 (mol/L)?
0,240 М
What net ionic equation is corresponds to a given molecular reaction?
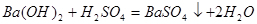
Ba2+ +2OH- +2H+ + SO42- →BaSO4+2H2 О
What net ionic equation is corresponds to a given molecular reaction?
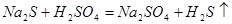
2H+ + S2- → H2S
Which net ionic equation is corresponds to a given molecular reaction?
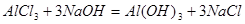
Al3+ + 3OH- =Al(OH)3
What pair of substances will interact with greater velocity if it is known, that concentrations of solutions of acids in all cases are equal?
Mg and HCl
What is Van’t-Hoff factor for KCl in aqueous solution?
2
What is Van’t-Hoff factor for Na3PO4 in aqueous solution?
4
Why it is advised to add ethylene glycol to water in a car radiator while driving in a hill station?
As an antifreeze to lower the freezing point of water
What is the expected Van’t Hoff’s factor ‘i’ value for K3[Fe(CN)6] ?
4
Write down the IUPAC name for each of the following complex [Co(NH3)5Cl]Cl2 and indicate the oxidation state and coordination number.
Pentaamminechloridocobalt (III) dichloride. Oxidation state of Co = +3; Coordination number = 6
What is the percent of MgCl2 by mass in a 0,01M salt solution that has a density of 1,09g/ml?
0,087%
Which of the following scientists was awarded the Nobel Prize in 1911 for the discovery of the radioactive elements, radium and polonium?
Marie Curie
Which of the following terms refers to the number of molecules or ions attached to a central metallic atom?
Coordination number
Which of the following pairs do NOT show similar chemical properties?
Fluorine-argon
Which of the following is NOT a general property of hydroxide bases in aqueous solution?
High solubility
Which of the following concentration expressions is defined as the number of moles of solute per kilogram of solvent:
Molality
Which of the following concentration expressions is defined as the number of moles of solute per liter of solution:
Molarity
Which of the following reactions involve NEITHER oxidation nor reduction?
Francium
What is the word that describes a substance that reacts with both strong acids and strong bases?
Amphoteric
Which of the following elements would form an acid oxide with the formula XO2 and an acidic compound with hydrogen with the formula H2X?
Sulfur
What volume of 1.5M NaOH is needed to provide 0.75 mol of NaOH?
ML
What type of reaction is indicated by the general form AB --> A + B ?
Decomposition
What type of reaction is indicated by the general form AB + C --> A + CB?
Single replacement
What type of reaction is indicated by the general form A + B --> AB?
Synthesis
What type of reaction is indicated by the general form AX + BY --> AY + BX?
Double replacement
When you heat magnesium in the presence of oxygen (in our air) in order to form magnesium oxide, what type of reaction is it?
Combustion
Which of these is the ground-state electron configuration for an atom of fluorine (atomic number = 9)?
1s2 2s2 2p5
What is the electron configuration for oxygen atom with charge -2 (O-2)?
1s2 2s2 2p6
Which among the following is not an example of irreversible process?None of these
Which of the following pair of liquids are immiscible?
Benzene + water
When sugar is added to water, what is the change observed in boiling and freezing points of water?
Calcium chloride
What is the equivalent weight of KMnO4 when it is converted in MnSO4?
M/5
What is the equivalent weight of KMnO4 when it is converted in MnO2?
M/3
What is the equivalent weight of KMnO4 when it is converted in К2MnO4?
M/1
Which of the following statement is correct?
Copper reacts with conc. HNO3 to produce NO2
What is the nature of electrolyte as Al(OH)3?
Amphoteric
Which metal is common between brass, bronze and German silver?
Cu
Which among the following is not a usage of standard hydrogen electrode?
Temporary hardness
What law is corresponds to this definition: “Equal volumes of all gases, at the same temperature and pressure, have the same number of molecules”?
Avogadro law
X ampere current is passed for y seconds through copper and silver voltameters. The metal that is deposited more is:
Ag
Disproportionation
An equivalent weight of oxidizer KMnO4 in a water neutral medium is equal ... (g/mol):
52,6
Applying the law of mass action to select the mathematical expression for the reaction rate 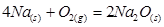 :
:
V = k [O2]
A solution containing 12.5 g of non-electrolyte substance in 175 g of water gave a boiling point elevation of 0.70. Calculate the molar mass of the substance. (Elevation constant for water EH2O = 0,52° Celsius)
53,06 g/mol
A potassium permanganate KMnO4 is a strong oxidizing agent in ................ medium
acidic
A potassium dichromate K2Cr2O7 acts as an oxidizing agent in ..................... medium
acidic
A concentration sodium phosphate solution is 17,0% (Na3PO4). The density of the solution is 0,900 g/mol. What is the molarity of the solution?
0,93 %
A solution with a pH of 12 is:
Strongly basic
All alloys contain this element if they are amalga. The element is:
Mercury
A double replacement reaction is likely to occur when :
two ionic compounds are dissolved in water
A catalyst can speed up the rate of a given chemical reaction by:
Дата: 2018-12-28, просмотров: 964.